The way I brought my background in physics to biology was by trying to represent a biological system as a data and information exchange system. Think of a telephone company, where you have millions of wires connecting individuals in a city. You can try and figure out what kind and how much information is transferred on those wires to come up with general principles about how the entire system works. We brought some very simple, very useful principles from information theory into biology, which helped us reconstruct the entire regulatory logic of a cancer cell, for instance. This was work that we did in 2005 with an algorithm called ARACNe, where we used the information that is transferred between a protein that regulates gene expression and the genes that are regulated to infer whether there was a direct regulatory interaction.
The cancer cell information revolution
Founding Chair of the Department of Systems Biology
- The entire mechanistic construction of a cell is like a computer, processing billions of pieces of information and trying to make decisions based on them.
- The term “personalised medicine” has come to mean something different: we’re actually personalising the treatment based not on the individual but on the molecular-level information that we get about the individual.
- Instead of trying to understand a disease at the single-gene or at the single-protein level, we’ve started to try and understand the entire mechanism.
- We now need a tremendous amount of cross-disciplinary expertise: people with backgrounds in physics, machine learning, engineering, mathematics and, of course, experimental biology.
Science simply got more complex
Science is changing because things that were relatively straightforward and simple to understand, because our knowledge had not expanded, have now become very complex. For instance, in the early days of biology, you could simply cut a gel on a protein assay and discover a new protein. It wasn’t easy at the time because the technology, of course, was very different from what it is today, but the things that we were looking at were individually simpler. Unfortunately, now, things have gotten much more complicated and we now have to understand how not just one protein but many proteins work together – and not just proteins but metabolites, RNA and all sorts of other molecules that work in the cells. And we have to understand how cells interact with one another.

Photo by isak55.
Applying the principles of information theory to biology
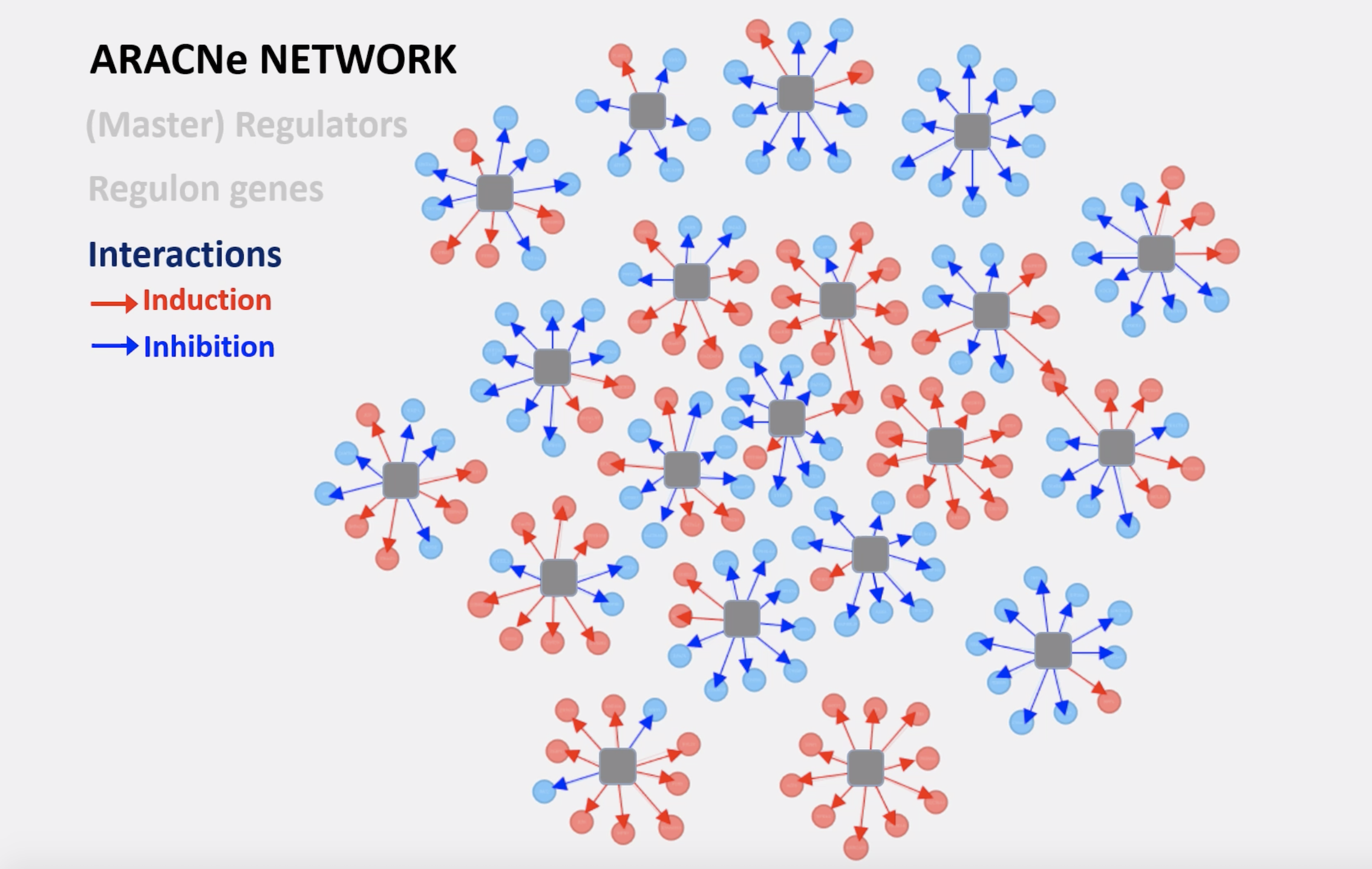
Image of the ARACNe Network courtesy of Dr. Heeju Noh.
Welcome to the cell information revolution
A cell is like a gigantic air conditioner. What an air conditioner tries to do is to keep the temperature in the room constant, despite wide changes in the temperature outside or even inside. If you had 10 people in a room, the temperature would go up or down. What the air conditioner tries to do is to use the information about the temperature to determine whether to turn itself on or off. That’s exactly what happens in a cell. All a cell is trying to do is to keep doing its function independent of all of the million perturbations occurring in the outside environment. You may be starved of nutrients. You may have dramatic changes in temperature. In fact, most biochemical reactions don’t work in the range of temperatures that cells experience. We’re talking about going from -40 degrees to +110 degrees. No chemical reaction would work in that time frame. You need a very complex biochemical environment to be able to make reactions work in that context.
Cells try to maintain homeostasis: equilibrium. We want to make sure that cells don’t change their function. For example, we don’t want one of our liver cells to become a neuron, or a neuron to become a B cell, because that would have pretty terrible consequences on our bodies. Most of the time, cells are preoccupied with trying to keep their state stable. To do that, they have to act as a computer, sensing information that is sensed by a membrane, for instance, or sensed by proteins that respond to heat signals or nutrient signals. Based on that information, they have to make a decision for us and change the biochemistry of certain reactions, to start making more of certain proteins that store information more effectively when we’re starved of nutrients. So, the entire mechanistic construction of the cell is like a computer, processing billions of pieces of information and trying to make decisions based on them.
The limits of “precision medicine”
The whole premise of “precision medicine” was based on the idea that by understanding the genetics of a cell, the genetics of the cancer cell in particular, we could make certain predictions about what drugs would be effective in that particular cell or whether an individual was likely to present with a particular disease. Unfortunately, what is written in our genes is only one tiny piece of information in terms of determining what cells do, because the information that is encoded in the gene is then processed by their regulatory machinery. It’s turned into RNA and then proteins and it’s the proteins that really do things in the cell and make it work in one way or another.

Next generation sequencing flow cell. Genetic engineering, cancer screening, medical technology and therapy. Photo by Elpisterra.
So, you can have cells that have identical DNA and yet do completely different things. A neuron in our brain would have the same DNA as a cell in our liver but do different things. The idea that has come out of precision medicine – which turned out not to be that precise after all – is that, if you have a mutation in a gene called EGFR, you can use a drug that targets the protein encoded by the gene and therefore kill the cells that are mutated. This simple concept has only paid off partially because most mutated genes today are not that responsive to the drugs that target the corresponding proteins – and with the ones that do, it’s very difficult to predict whether the patient will respond.
For example, with a melanoma in a common mutation in a gene called BRAF, there are very effective drugs that can target the corresponding proteins and shut down its activity dramatically. And patients have striking responses. Unfortunately, those responses only last for a few months and the disease then comes back even worse than before.
What can we do to improve patients’ reactions to treatment?
Instead of trying to understand a disease at the single-gene or at the single-protein level, we’ve started to try and understand the mechanism as a whole. Think about a complicated watch. We’re trying to understand how the gears, pulleys and connections in a watch make the entire mechanism work. This enables us to zoom in on the specific gears that may be malfunctioning. This is something that has been very difficult in biology because you need powerful supercomputers to model the complexity of a cell.
The benefits of modelling the complexity of a cell
Think about it. Twenty thousand genes; maybe more than a million protein isoforms. It’s a very, very complicated system – and it’s not just one cell. There’s around one billion cells in a tumour. You can detect it. So, the idea of going from precision medicine, which is based on individual genes, to predictive medicine, which is based on understanding how all the gene products work together in a cell, is ongoing in the field right now. This is the same for medicine. We’re trying to turn medicine from an empirical base – whereby if you have a certain symptom, you need a certain type of cure – to something where the same thing can be done, using a variety of molecular-level data that can be gathered about the patient for more precise and more accurate predictions.
Healthy cells. Image ©DarwinHealth.
What is personalised medicine?
The concept of personalised medicine is a misnomer, because the way people decide how much of a particular drug you should get – for example, if you have a cardiovascular problem – is by trial and error and by adjusting these levels. So, each individual may get different amounts of the same drug. Personalisation has long been part of the medical community approach. Some physicians get offended by the idea that we’re now doing personalised medicine because, in reality, this has been going on forever. I think the term “personalised medicine” has come to mean something different, which is that we’re personalising the treatment based not on the individual but on the molecular-level information that we get about the individual. This can be extremely complex.
One extreme of this paradox is that we may then need a different drug for each patient, which, of course, is not going to work because drugs have to undergo clinical trials. So, how do we do a clinical trial with a single patient? There are ways to get around that, but it’s certainly not the way we think that the problem will be solved. The paradox will be solved by studying enough people at the individual level to then make generalisations that cover a broad range of individuals with similar characteristics.
A billion different diseases
Now that we can do things at the single-cell level, a disease like cancer for a single individual is no longer a single disease. It’s literally a billion different diseases because there are a billion different cells in that initial mass, and every one of them has a slightly different range and repertoire of mutations and behaviours. Within one tumour, we may find two, three, sometimes eleven different subpopulations of the tumour that require different drugs. So, there is a personalisation that goes beyond the individual, to the subset of cancer cells that are producing ill-effects in the patient. In some cases, you can even focus on the population that may be the one that will maintain the entire tumour. Therefore, if you just killed that population, the entire tumour would flop and die. There are many different levels of personalisation, many different levels of precision and predictive medicine, but we certainly don’t want a situation where every single patient requires a different treatment.
Cancer cell. Image ©DarwinHealth.
The consequences for biology
The panorama, the schematics of our biological systems work, has become so complex that the original approach, which was just to devise relatively straightforward assays that could then tell us a response and answer to a specific question, no longer apply. We now need a tremendous amount of cross-disciplinary expertise: people with backgrounds in physics, machine learning, engineering, mathematics and, of course, experimental biology. It’s only when you put these teams together, you realise today’s potential of the science of biology, and of biology-powered medicine.
What makes a cross-disciplinary scientist?
A lot of what makes a scientist cross-disciplinary is the ability to bridge these vocabularies and bridge the understanding of the underlying phenomena that are brought forward by the different disciplines. Here in my department, our mission is to create scientists who no longer think of themselves as being computational biologists or experimental biologists. We want them to think of themselves as “just” biologists that use any possible tool or trick of the trade or methodological approach that will help them address the important question they’re asking. The focus goes from the methodology that you’re using to the question that you’re asking. We are ready to develop whatever methodology – experimental, computational, engineering-based or mathematically based – is required to solve a particular question. The real shift has come from working on technologies that are very narrowly focused to working on questions that are very complex and difficult to address using just one technology. And so, we need to bring together the expertise of many different researchers working in very different and complementary fields.
Discover more about
the cancer cell revolution and the future of science
Margolin, A. A., Nemenman, I., Basso, K., et al. (2006). ARACNE: An Algorithm for the Reconstruction of Gene Regulatory Networks in a Mammalian Cellular Context. BMC Bioinformatics, 7, S7.
Alizadeh, A. A., Aranda, V., Bardelli, A., et al. (2015). Toward understanding and exploiting tumor heterogeneity. Nature Medicine, 21, 846–853.
Thorsson, V., Gibbs, D. L., Brown, S. D., et al. (2018). The Immune Landscape of Cancer. Immunity, 48(4), 812–830. e14.