I was recently at a meeting where Michael Bishop highlighted a paper from a group in Pisa in the 1960s or 1970s where they categorically stated that cancer could not possibly be due to a mutation in the genome.
Decoding the full story about cancer
Founding Chair of the Department of Systems Biology
- In our lab, we are focusing much more on developing treatments that will target transcription factor activities as opposed to targeting genes that are broken by mutations.
- We thought, until very recently, of cancer as sort of a monolithic object, where the cells of the cancer cell are all doing the same thing – and all doing the same wrong thing. It turns out that that’s not the case.
- If we found recipes to effectively target primary cell populations, then we could deal with any tumour within one patient.
- By combining genetics and immuno-oncology systems approaches, we hope to carve the pie of cancer into smaller and smaller slices and make therapies that work.
Changing our perceptions of cancer
For a long time, cancer was thought to be a sort of random event and it was not clear that the etiology of cancer was associated with changes in the genes.

Photo by 18percentgrey.
Right after that, there was major work by the pioneers of cancer – Harold Varmus et al. – who showed us that cancer, or at least a certain number of cancers, were associated with very specific reproducible mutations in a set of genes called oncogenes. These oncogenes have a property that when they were mutated in that fashion, by a mutation that increases the activity of the proteins that is encoded by gene, then cancer could ensue.

Harold Varmus, Director, National Cancer Institute (2010). Wikimedia Commons. Public Domain.
That really changed the face of how we started thinking about and studying cancer. There was a major shift of the entire research field to studying the genetics of cancer, and an entire field of cancer genetics was born from that revolution. For a long time, we held onto the hope that understanding the genetics of cancer would actually help us completely solve the problem. Things are much more complex and complicated because it turns out that cancer derives from so many different potential patterns of gene mutations and also from signals from the environment.
The interactions of broken genes and signals from outside of the cells
One particularly important set of signals, the endocrine signals – the typical oestrogen and androgen signals – are responsible, for instance, for breast cancer and prostate cancer in men and women. So, interactions of broken genes and signals from outside of the cells really creates the prerequisite for the cancer cell to develop, differentiate and grow and to start accumulating even more mutations. There’s also a very precise set of steps the cancer goes through because the initial mutation is almost never tumorigenic. So, even in the most powerful oncogene, a gene called KRAS, it’s not sufficient to have a mutation because the cell already knows that if you have a mutation in KRAS, you’ve got to stop everything you’re doing and put that cell into a senescent state so that it will no longer be a problem. Recently, in our nevi, we have a lot of mutations in KRAS or BRAF that are not tumorigenic, because they’re kept at bay by senescence. You need not just one gene, but many genes, to be broken and so, typically, the combination is an oncogene and a tumour suppressor.
The full story about cancer
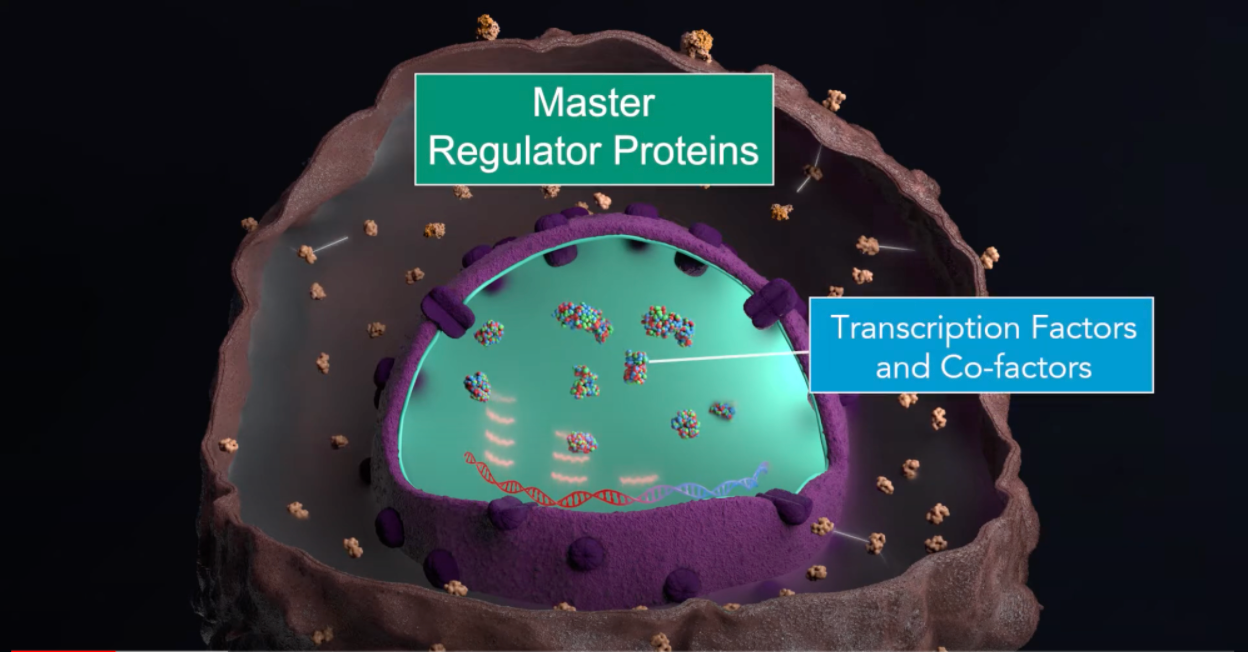
This is basically the foundation of cancer. Now that we have discovered that the individual genes don’t really tell us the full story – and there are many patients with cancer where we can’t even find what we call a driver mutation – and that there has to be something more complex, which is exactly what we’re looking for, for instance in looking at the dysregulation of transcription, we find that the transcription factors and cofactors that are dysregulated in cancer that are acting in an abnormal way in cancer cells are extremely more conserved and reproducible than the specific mutations that induce the cancer state. This is why, in our lab, we are focusing much more on developing treatments that will target these transcription factor activities as opposed to targeting genes that are broken by mutations.
What cancers have in common and how they differ
We thought until very recently of cancer as sort of a monolithic object, where the cells of the cancer cell are all doing the same thing – and all doing the same wrong thing. It turns out that that’s not the case. Cancers have cells that are in multiple states. Think of those states as being the three primary colours on a palette. You can then generate any colour you want by simply mixing those colours in different ratios. What happens is that cancers have looked very different in many cases because they have had these primary colours that form them mixed together in very different ratios within each individual. And so, the individuals look different from another one while the actual primary state of the cancer cells is exactly the same. This is a very important concept because what we find is that, even after treatment with drugs, the cancer cells are not really changed, overall. If you look at the entire tumour and you average across all the cells, the tumour will look very different. It’s simply because within the same patient, the ratios between the populations of cells that are in one state versus another have changed.
What these ratios mean to cancer therapy
This tells us that if we actually find recipes to effectively target those primary cell populations, then we could actually deal with any tumour. Now, this is within one patient. When you extrapolate this concept to all the patients with different cancers, what emerged from a recent analysis that was just published in Cell is that we could only find 24 major modules of master regulators. Remember, these are the proteins that control the expression of the genetic programmes that make the cell work in a certain way or another. These 24 blocks were turned on and off in different ways, in different tumours, but they were pretty much exactly the same blocks and pretty much working in the same way in different tumours. It’s a bit like a Lego recipe, where you have different pieces. But these pieces could only be assembled to 24 different types of pieces in different ways.
In some cases, you may find that a particular module being turned on is bad for a tumour, but in a different tumour, another module being turned off is bad for it. So, it’s really the combination of these modules together. That again gives us hope because if there are really only 24 modules – which remains to be validated and consolidated – you could think of generating drugs that can target each one of those 24 modules. You would then have a finite recipe of things that you need to develop in order to not make sense of just one tumour, but potentially every tumour.
This idea that we can generalise and conceptualise cancer as driven by more universal principles is very important, because the last few years have given us the impression that every cancer cell and every tumour is different, which has left us wondering how to generate drugs we can validate in a cohort if all the patients are different. This is the leitmotif of what we’re trying to do in the lab.
The power of oncotecture
Oncotecture – A model of cancer cell dysregulation. Image © DarwinHealth.
Think of the architecture as being an hourglass. You have lots of grains of sand on the top and you have lots of grains of sand on the bottom. But they all have to go through a bottleneck, and that bottleneck is the dependency of the order of that particular mechanism. If you put a plug in the bottleneck, you’ll have many grains of sand on the top that will no longer make it to the bottom. If you think about Manhattan, there are only a handful of bridges and tunnels. If you want to block the entire flow of people getting in or out of Manhattan, you just block those foundational points.
The oncotecture concept is saying that even though cancer is dysregulated by an almost infinite number of mutational patterns – we’ve estimated a potential number to be larger than the number of atoms in the universe – the actual ways in which these patterns coalesce is very, very limited, and they do so through these bottlenecks. And so, what is really repeated in reproducible cancers is not necessarily the individual mutations, but rather the way in which these mutations are integrated by the logic of the cell. Think of it as a computer logic and brought to bear on this very tight bottleneck where everything is coming together. And that bottleneck is this group of master regulators that we call a tumour checkpoint. Think of it upstream. You have all the mutations in the bottleneck. You have the proteins that integrate the mutation. Then, downstream, you have the transcriptional state of the cells: which genes are being expressed, and which genes are not.
Creating new drugs that work against cancer
Our lab is fully integrated. We have people working at a pure algorithmic level, in some cases, not even knowing very much about the underlying biology. Then there are people that are MDs and pharmacologists that work with patients or develop new drugs. In order to have that level of integration, you need a variety of different technologies at your call. The ability we have built through the department allows us to do very large-scale perturbational experiments where we can, for example, take a number of different cells that are models for tumours that we want to study and perturb them with drugs and then read out what has happened to that cell at the gene expression level so we can effectively predict the proteins that went up and the proteins that went down when you put the drug in.
This is very important because it’s essentially an impedance match. These proteins are bad because they’re activating the cancer. These are the proteins that have been abrogated. All you need to do is to essentially combine and figure out what drug is inactivating not just one but all the bad proteins that are being activated in the tumour or inactivated in the tumour. This, you can only do using very sophisticated and expensive robotics approaches; in fact, in some cases, we had to develop technologies completely from scratch. We now have a technology called PLATESeq that was developed by the Peter Sims Lab in collaboration with mine, which allows us to do the transcription of cells for about $11 per sample, whereas normal transcription profiles cost about $200. This is about to change the scope of what we can do in terms of drug perturbations.
Treatment choices for cancer patients

Photo by Gorodenkoff.
Patients today have three choices. The tumour can be resected surgically; that’s all. The vast majority of cancers, but only if it happens before the tumour has metastasised, have a genetic alteration that can be targeted pharmacologically. That happens in a very small number of cases, in about maybe 15% of patients, and most of those patients will eventually relapse. They can undergo immuno-oncology treatment because their immune system can be reawakened. Also, those patients represent a very small number compared to the full gamut of cancer and, in many cases, those patients relapse. So, this leaves an enormous number of patients that essentially have no solution. What we’re hoping to do is, by opening the cancer box and figuring out the entire set of mechanisms that make this bad watch work, we can figure out solutions for patients that have none.
A complementary approach
We have several examples of patients that have been treated with the drugs that have been predicted by our analysis who have had really striking responses. Right now, what we are doing with seven clinical trials that are running is trying to see whether we can bring this into a very rigorous clinical validation, where we can demonstrate in which cases these approaches work or don’t work. This is not a magic bullet. Just like everything else that has been done in cancer, it is an additional and complementary approach. But we hope that by combining genetics and immuno-oncology systems approaches, which is exactly what Columbia University is doing, we can carve the pie of cancer into smaller and smaller slices and really make therapies that can work for those patients.
Discover more about
oncotecture and cancer cell mechanisms
Califano, A., & Alvarez, M. J. (2017). The recurrent architecture of tumour initiation, progression and drug sensitivity. Nature reviews. Cancer, 17(2), 116–130.
Kushwaha, R., Jagadish, N., Kustagi, M., et al. (2014). Interrogation of a Context-Specific Transcription Factor Network Identifies Novel Regulators of Pluripotency. Stem Cells, 33(2), 367–377.
Califano, A., Butte, A., Friend, S., et al. (2012). Leveraging models of cell regulation and GWAS data in integrative network-based association studies. Nat Genet, 44, 841–847.