To be able to intercept disease is a future way of seeing medicine, where you don’t have to wait until you are in the worst situation. For example, in the case of cancer, the idea would be to intercept the risk or the transition towards metastasis. If you know there is a risk – if you can look at a population of cells, and you see one that is a danger and that may give rise to metastasis – then you can prevent that cell from going in that direction. You can put it back on the right path. That would be a way to prevent adverse phenotypes and adverse situations for people.
Understanding the organisation of our DNA to intercept disease
Professor of Molecular Biology
- The organisation of DNA is important to fit DNA within the nucleus and to ensure that certain regions remain accessible or hidden.
- Chromatin is a complex of protein and DNA which is key to the functional organisation of DNA.
- A number of alterations at the chromatin level have been associated with cancer cells. One future research goal is to be able to intercept as well as treat disease.
Why our DNA has to be organised
Understanding why the organisation of our DNA matters is quite simple. First, think about having two metres of DNA within the nucleus of each cell, which is a few microns in diameter. This needs to be packaged so that it can fit inside.
The DNA has to be organised to fit into a small volume, and this can’t be done at random. You need a high degree of compaction while also keeping certain regions accessible so they can be used in certain cells, and others hidden for later use or not to be used at all. It’s this functional organisation in the nucleus that matters.
The role of chromatin
Chromatin means colour, and it’s a term that was coined by Walther Flemming. Chromatin is the material in the cell that could retain stains like aniline that were used at the time. By using this staining, Flemming was able to look at this material and see how it was changing in shape during the cell cycle. The most extreme version of this is the chromosome, which you can see when the cell divides to give the same number of chromosomes to the two daughter cells.
Molecular understanding of chromatin came later with work by Arthur Kornberg and Pierre Chambon. They found that chromatin is a complex of protein and DNA, which has a basic unit where DNA is wrapped around a core of protein, called histones. When viewed with electron microscopy, it looks like beads on a string because it regularly repeats. This organisation can then adopt further types of organisation, but this first level is key to enabling organisation in the nucleus.
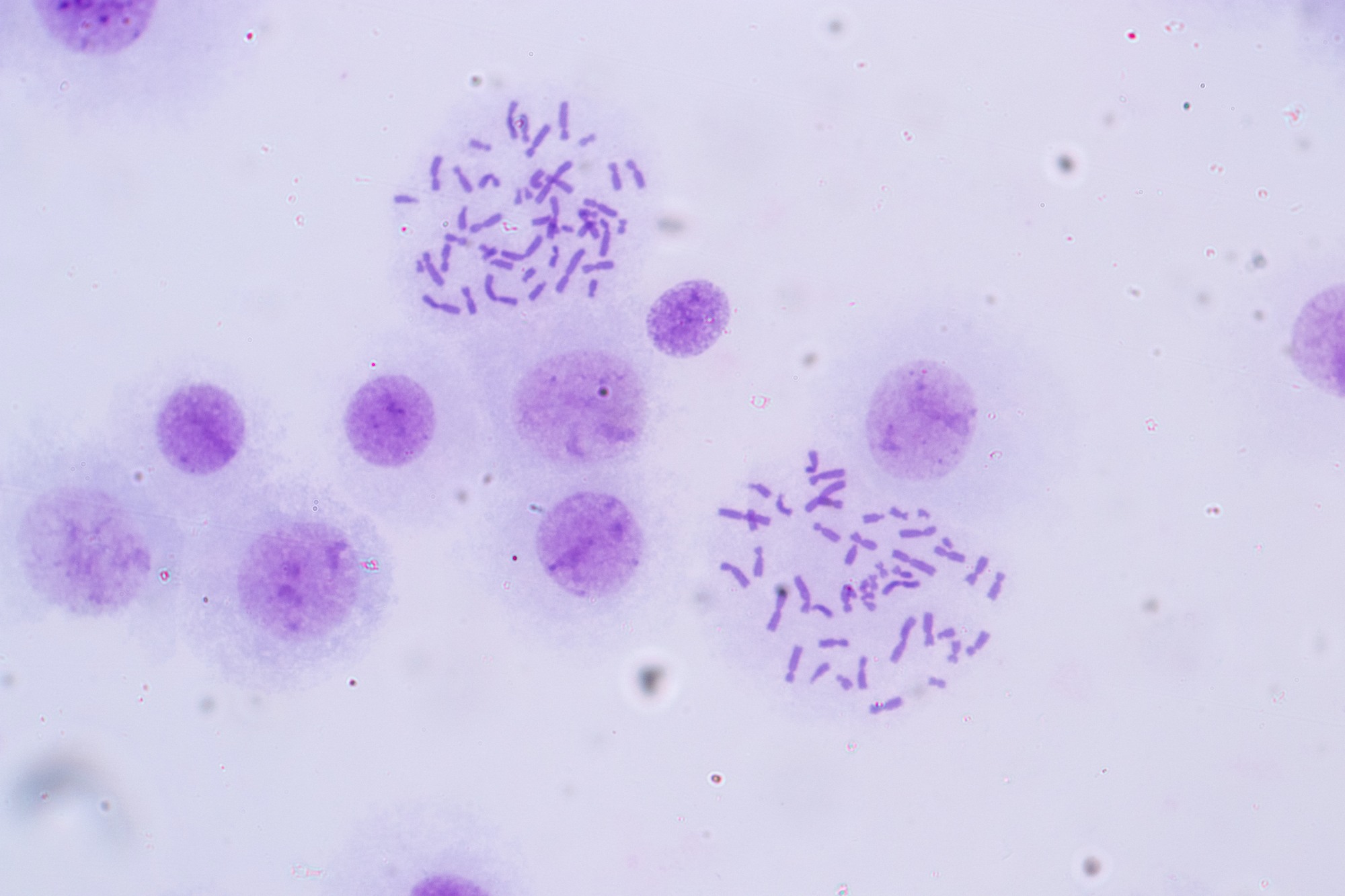
Human chromosomes under the microscope in lab. By Rattiya Thongdumhyu.
Organisation and function
People realised that there were mutants in the histones, which are the proteins that are the very constituents of these basic units, that could impact on the transcription or the genome function of certain genes. That linked organisation with function.
Since then, a lot of research has continued to try and understand: how do you organise or put histones into chromatin? What about the assembly – how do you take them off? How do you expose a region so that factors can interact with the DNA easily? Or how can you prevent potential interaction? How do you dynamically work with this in the nucleus? This has been an area of intense work, and many people have contributed over the years.
Key research questions
There are several key topics to consider when you look at the nucleus and take into account chromatin as a level of organisation. First, if you think about any process that occurs on DNA, it doesn’t actually occur on naked DNA; it occurs on chromatin. So one of the first things people studied is how transcription works with in vitro systems, trying to reproduce with DNA and transcription factors the machinery to get that to work.
But how does it work when you’re on chromatin? How does it work when you get back to something that is closer to the physiological context? One thing that was learned is that the presence of nucleosome could act as a barrier. So then you had to understand how to deal with this, because you have to be able to act on the material.
The second issue was replication. This is another thing that has to happen on DNA, but it’s happening on chromatin. And so how does the replication machinery deal with this complex architecture in the nucleus? How does it duplicate both the DNA and its organisation? How does it go to disrupt it first and then to rebuild it? If we think about DNA repair, we face the same questions.
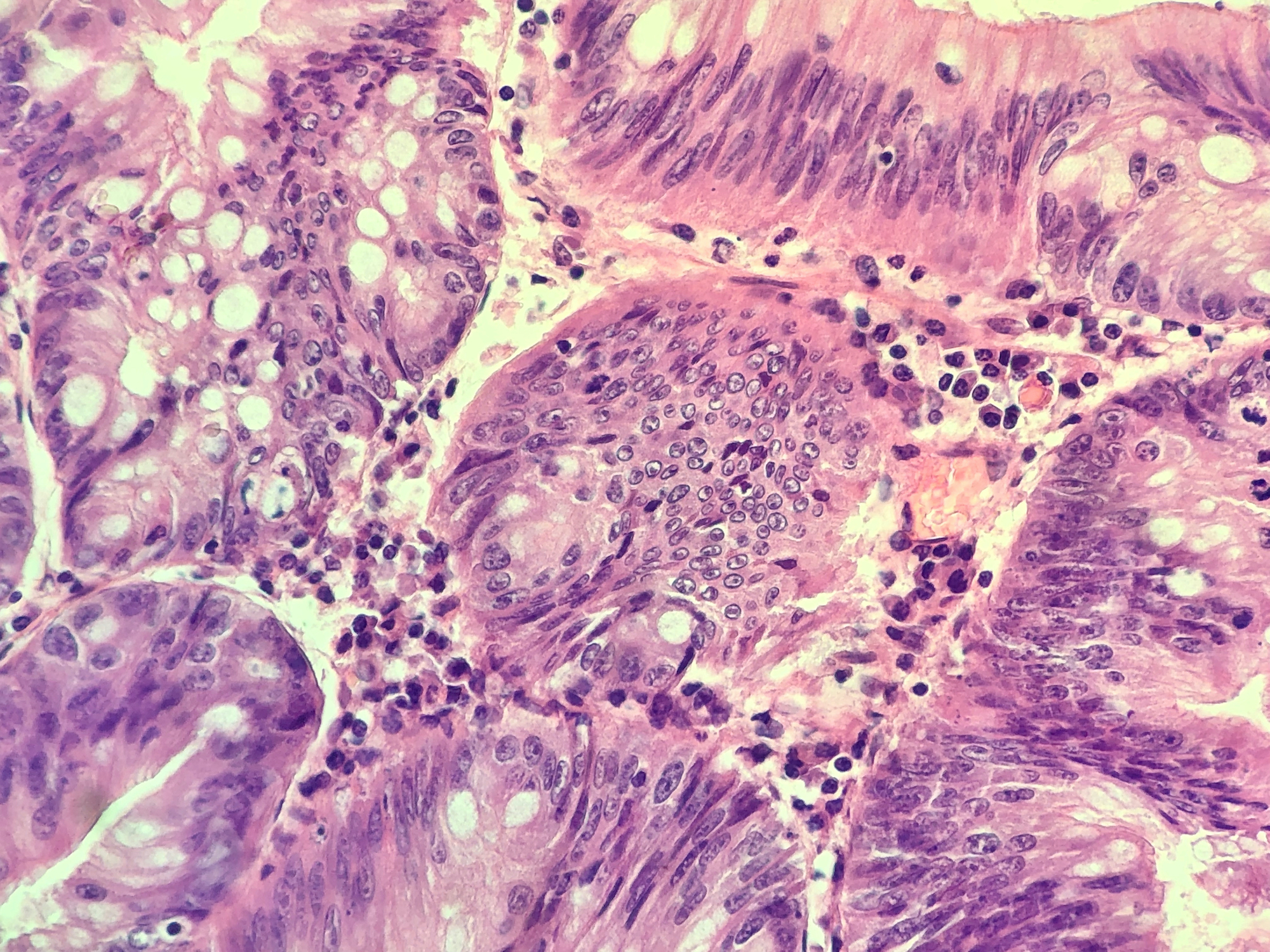
Tissue showing lots of different types of cell with the nucleus detailed in violet and cytoplasm in pink. By Ilariamulas.
Chromatin dynamics and cancer
What can go awry in cancer cells? In this context, a number of mutations have been analysed that affect histones themselves. This has given rise to the concept of oncohistones. It showed that through mutation, this component of chromatin could contribute to the development of disease.
Other mutations have also been found. There’s a significant number of factors called remodelling factors. These are complex and important to rearranging the nucleosome. There are also changes in the balance of the dosage of factors that are important to escorting the histones, like histone chaperones.
These are just some examples of entry points that can help make connections. You start to understand that changing or altering aspects that affect the organisation in the chromatin component of cells could be important in the context of cancer. Studying this gives you a glimpse of how you could potentially change this.
Developing new treatments
We’ve seen a number of alterations at the chromatin level that have been associated with cancer cells. Once you identify a problem, you have a target. A number of these targets can be druggable with an enzyme that you can potentially inhibit. There have been a lot of so-called epi-drugs, which could target many of the enzymes that can modify chromatin modifiers themselves, and which could also inhibit remodellers at the level of their subunit that is key for their activities.
These are some ways to address the issue. But usually they are not used alone. Very often they are used in combination to sensitise the cancer cells so that they are more prone to a second agent that will then be inflicted. It’s like an Achilles heel; you are trying to make your opponent much more responsive to a second attack.
Future research challenges
We want to better understand how the different levels of organisation of chromatin in the nucleus can be dynamically rearranged in different cells over time and depending on the environment. We’ve been learning a lot at the level of the basic unit, the nucleosome. But we’re interested in how this impinges on the higher organisation within the nucleus, and how these dynamics work at different scales and different times. It is going to be a challenge; we will be using tools with advanced imaging and the capacity to learn about a single molecule in detail.
We also have to zoom out and integrate how that functions in individual cells in an organism – not only in normal situations but also in pathological systems. New tools will be important for this as well, including the possibility of using organoids – mini-organs that can mimic parts of ourselves – as well as artificial intelligence to analyse large amounts of data.
Intercepting disease
Combining information from different angles will be necessary, and that will certainly be a worldwide team effort. I hope that with this approach, by linking up with partners that are interested in the medical angle, we can take things forward to gain a better life and perhaps intercept disease. This is the dream – to discover problems before they bring adverse symptoms.

Medical consultation. By Sheff.
Discover more about
genome architecture
Morel, D., Jeffery, D., Aspeslagh, S., et al. (2020). Combining epigenetic drugs with other therapies for solid tumours — past lessons and future promise. Nature Reviews Clinical Oncology, 17, 91–107.
Müller, S., & Almouzni, G. (2017). Chromatin dynamics during the cell cycle at centromeres. Nature Reviews Genetics, 18, 192–208.
Prendergast, L., Müller, S., Liu, Y., et al. (2016). The CENP-T/-W complex is a binding partner of the histone chaperone FACT. Genes & Development, 30, 1313–1326.