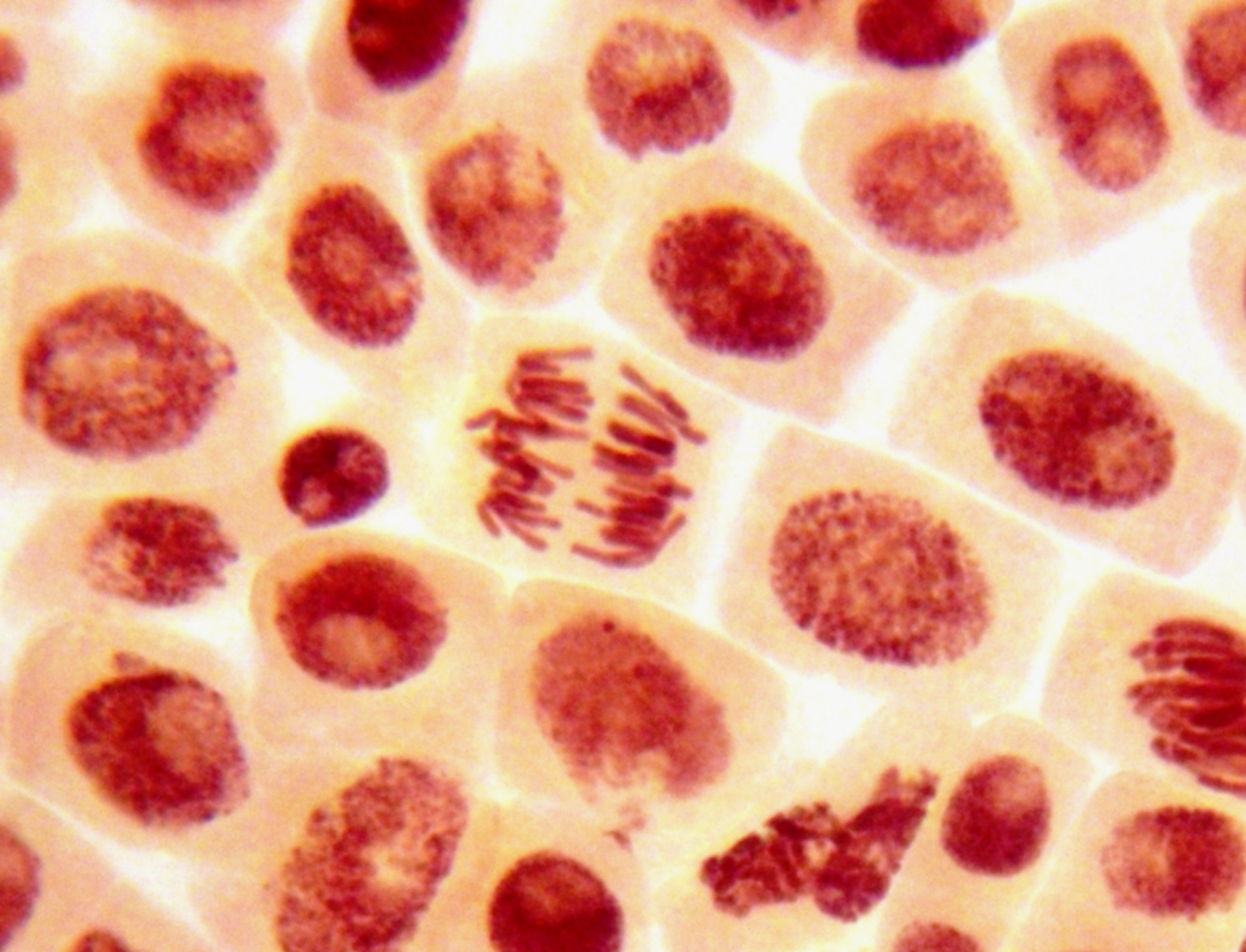
Stem cells are the cells in your body that are responsible for maintaining your tissues throughout your life. For example, in the blood, some cells only live for a few hours. If they weren’t replaced by stem cells, you would run out of blood cells. That’s a definition for stem cells in our bodies, but there’s a whole other type of stem cell that is equally important: stem cells that are made in the laboratory.
Originally, they were called embryonic stem cells because they were made by culturing cells in the lab from discarded embryos, from in vitro fertilisation procedures. In about 2007, Shinya Yamanaka’s work in Japan showed that you could convert adult stem cells into cells that were like embryonic stem cells – called induced pluripotent stem cells, or IPS cells. What’s important about embryonic stem cells, or IPS cells, is that if you give them the right instructions, they can be converted into any tissue in the body.
Stem cell therapies
Stem cell therapies are treatments which are designed to alleviate disease by manipulating stem cells. The classic, and still very important, form of stem cell therapy involves transplanting stem cells from the blood of one person into somebody who is in need of that, so blood transfusion is the most classic cell therapy.
Depending on the kind of stem cell and the disease indication, there are many different ways in which stem cells are used. A more experimental example would be taking pluripotent stem cells – IPS cells – turning them into cells that function in the back of the eye – cells of the retina – and transplanting them, to repair diseases that result in blindness.
A further application of stem cells would be to kill cancer cells. The basis of some of the new immunology-based cancer treatments is that you do a transplantation of a T cell engineered to seek out and kill cancer cells. So, in terms of stem cell therapies, there are probably as many types of therapies as there are tissues in the body or diseases that we know about.
Challenges associated with stem cell therapies
There are a number of different challenges of stem cell therapies, and it might surprise people to realise that they’re not all about pushing the frontiers of science. For example, I’ve described how blood transfusions are a classic way of using cells to treat blood loss. In this situation, the challenge is one of logistics: cells are collected from a person in one place and they need to be delivered to the person that needs the donation in another place.
Another kind of challenge is one where you have the proof of principle or concept that the therapy will work, but there are practical reasons why it’s not used. A classic example of that would be that in the early 1980s it was shown that you could take a piece of skin from somebody who had suffered a burn injury, expand it in the laboratory and graft it back onto the patient. This is a treatment which has been shown to work, to be safe and to save lives. However, the logistics of having the facilities to expand those cells in sterile conditions and the technical expertise to do the grafting have meant that from a cost point of view, it’s really expensive.
When we think about safety issues for new therapies, there are a number of ways to consider that. If a transplant of cells involves your own cells being expanded in the laboratory and transplanted back into you, you can be confident that your immune system will tolerate the treatment. However, if you are transplanting cells from one person to another, it may be necessary to suppress the immune system. Of course, organ transplantation has been around for a very long time and people do well on the right cocktail of drugs that suppress the immune system for many years, although they have side effects.
So, one aspect of safety is how the stem cell transplant is going to interact with the immune system. Another issue which has concerned investigators from the beginning is taking a treatment which involves IPS cells. For example, if you don’t get 100% of the cells converted into the beneficial cell type, is there a risk that the cells which remain in the pluripotent state might proliferate over years in an uncontrolled fashion and lead to cancer?
If we think about safety issues for stem cell therapies, it’s very helpful to look at a sister field of research, the field of gene therapy, which involves correcting defective genes or replacing missing genes. That field is potentially older than the stem cell field, and the safety issues that have had to be overcome are well worked out.
Surgical waste skin
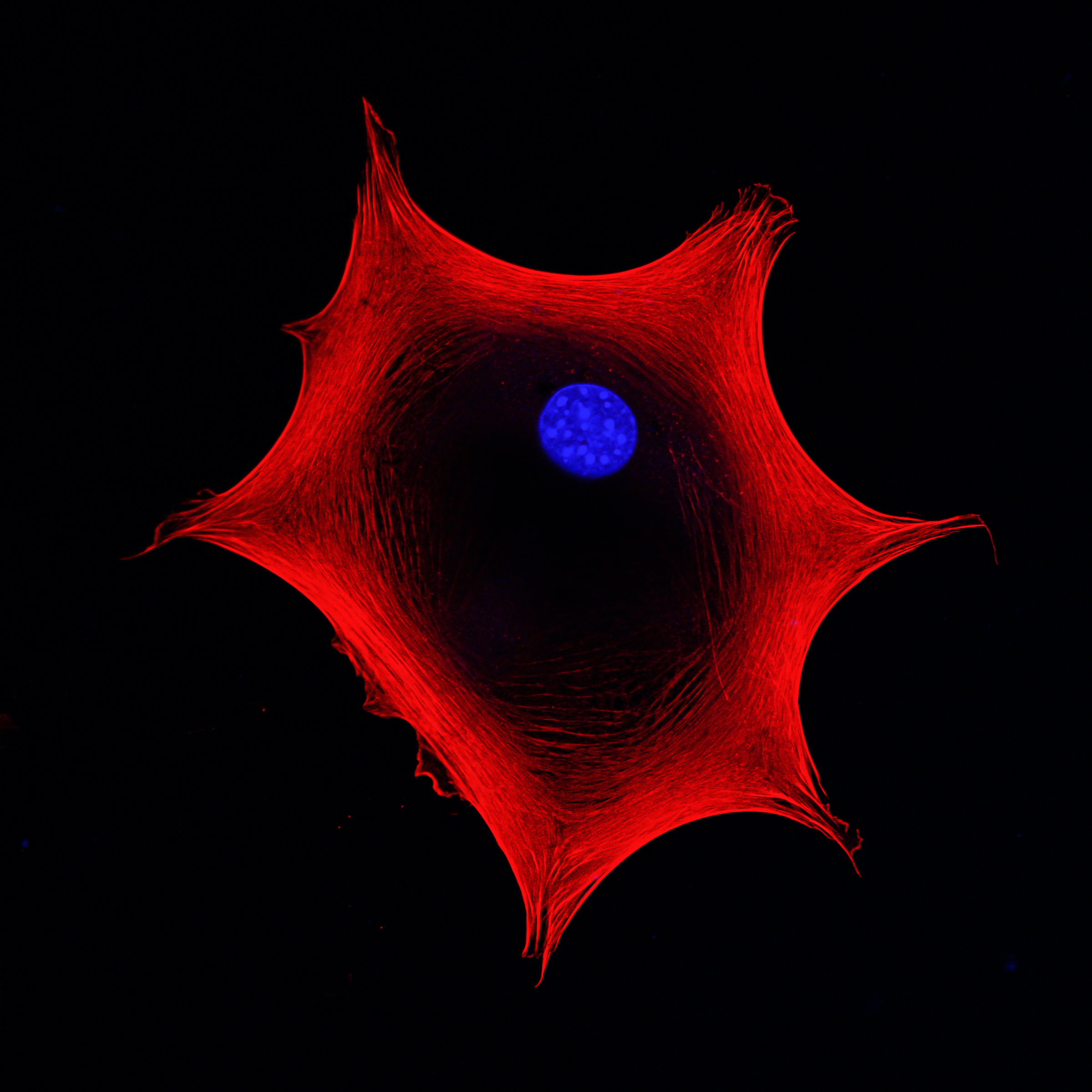
In my Centre at King’s College London, we carry out discovery research on a number of different stem cell types. We have a toolkit which allows us to expand stem cells in different ways. One example is where we study cells from human skin. For this, we simply need to receive a skin donation. It’s usually what we call “surgical waste skin”: somebody may have wanted cosmetic surgery, for example a “tummy tuck”, as we say in the UK. They consent for their skin to be used in the laboratory. We separate the different cell types using enzymes to disaggregate the skin and then we use the culture medium of food to expand the cells and keep them as close as possible to the nature of the cells that came from the original skin.
Working with a goal in mind
Our approach to working with stem cells in the lab and expanding them is informed by the end goal. As an example, we’ve discovered some cells in human skin that we believe could be beneficial to treat scars, the long-term scars that people would be familiar with. Having made that discovery, the next stage is to make absolutely sure that we have protocols in place which we can follow rigorously so that we always get the same outcome. At that point, we move from the discovery phase to the process development phase.
In a different lab setting, however, the question might be why somebody who has a particular genetic disease suffers from kidney problems in adulthood. In that instance, we would follow different protocols to make kidney organoids and then we would try and stress test the system to find out whether there are any experimental conditions that would reveal why a patient suffered kidney damage in adult life.
So, when we work with stem cells in the lab, we always have a clear goal in mind, but that goal will determine whether we’re following a very straightforward step-by-step process, which must always be the same, or whether we look at the results we’ve obtained and then systematically vary the condition to see if we can test hypotheses we have about disease mechanisms for example.
One of the things I’ve learned during my career is that my predictions are almost always completely wrong. I would never have predicted that it would be possible to turn an adult tissue stem cell into an induced pluripotent stem cell. I definitely, having seen the gene therapy field in the doldrums for a long time, would never have predicted that a set of genes which bacteria used as a primitive form of immune system could potentially give you a very efficient way of gene correction.
My only prediction, and it’s really an aspiration rather than a prediction, is that I truly believe that what we call the field of advanced therapies – so that’s cells, genes or cells plus genes – is going to provide benefit to patients over the next 5 to 10 years. I couldn’t predict in what way or what indications, but I am very confident that the right combination of skills, people and interests are committed to pushing this forward as a field.
Changing cancer treatments?
I’ve spent quite a lot of time in my research career studying cancer, most recently cancer of the mouth and cancers that affect the tongue. These are cancers that have been difficult to treat, but we know a lot more about them now than before. I believe that for those kinds of cancers, treatments based on immune cells will be part of the armoury, the set of weapons that we have to deploy for cancers.
The classic treatments for cancer are surgery – just removing the tumour – what we would call radiation; and, over the past 50 years, drugs that kill the cells. I believe that immune therapies which involve cells or other types of immune system regulation are already established as an important weapon. To some extent, the challenge now is to work out, in advance, which combinations of those weapons should be deployed and at what stage.